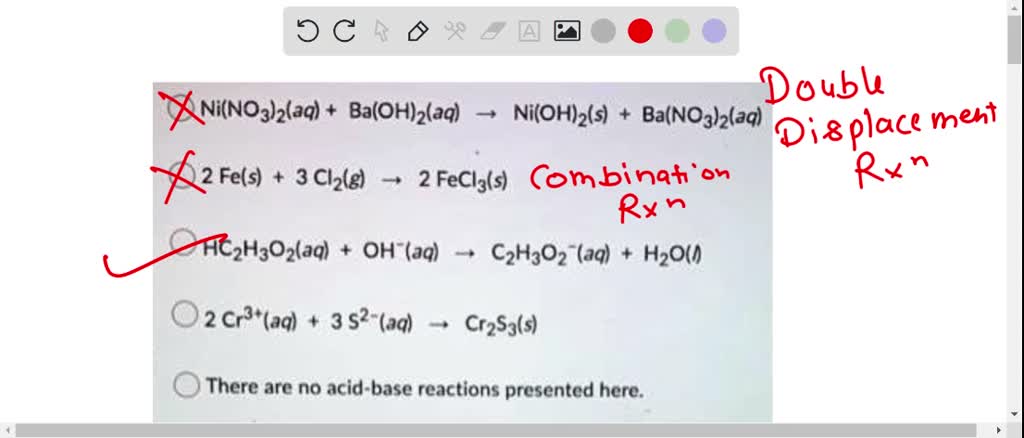
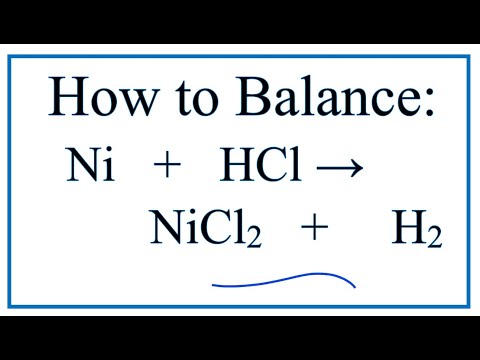
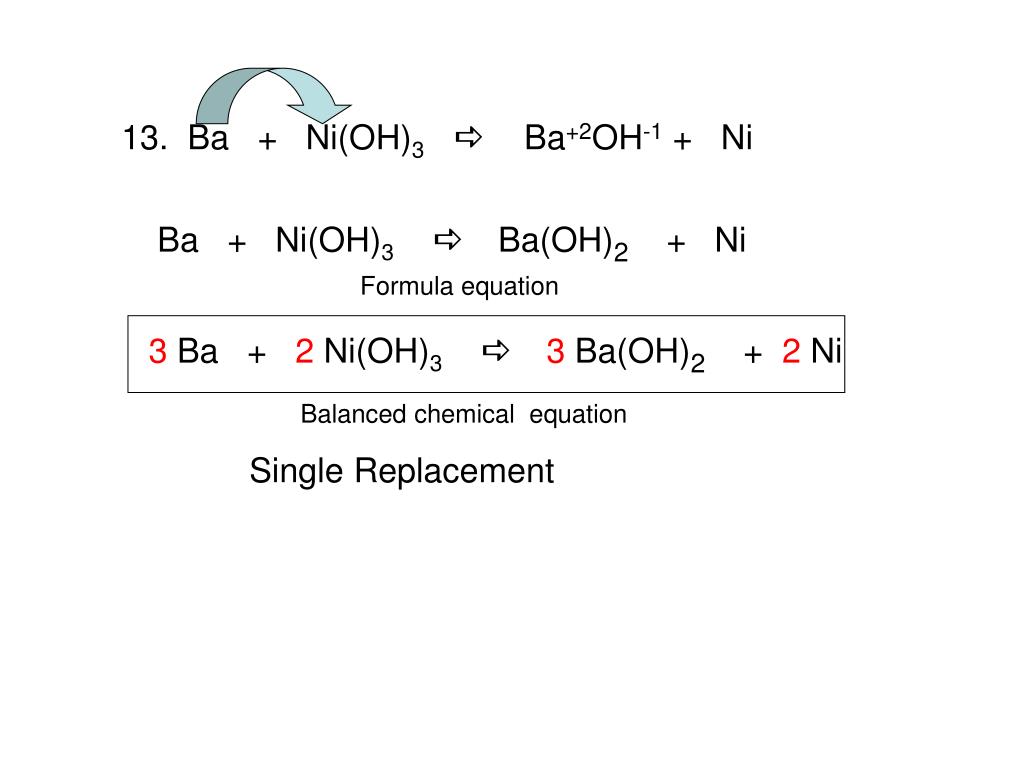
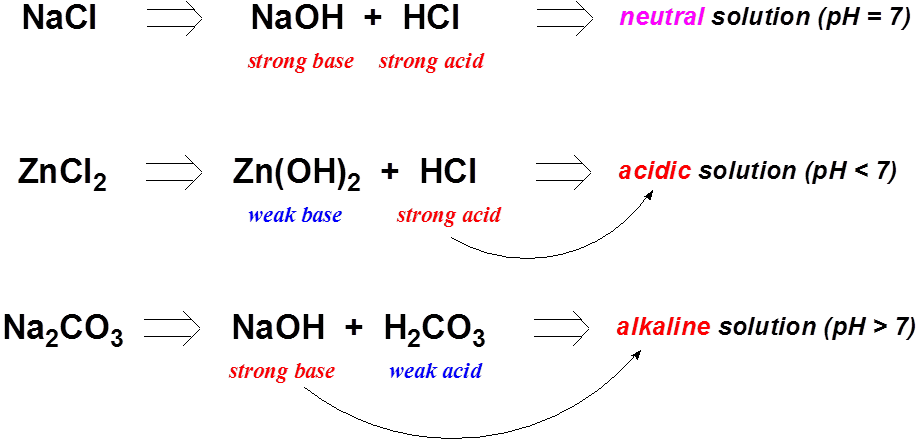
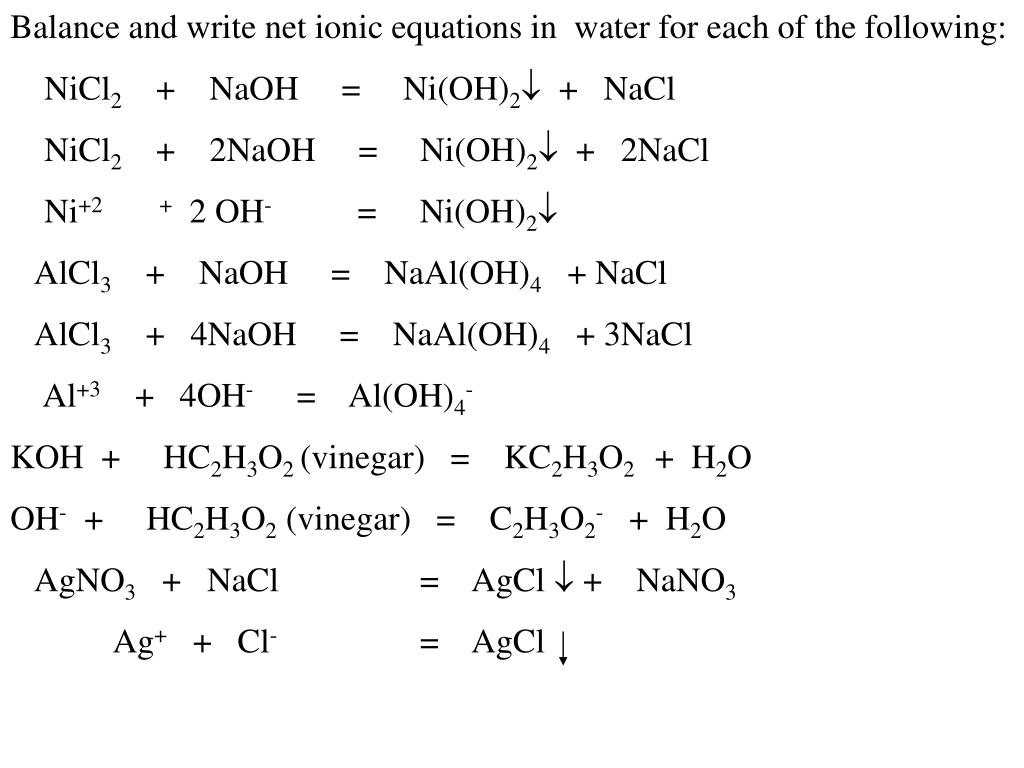
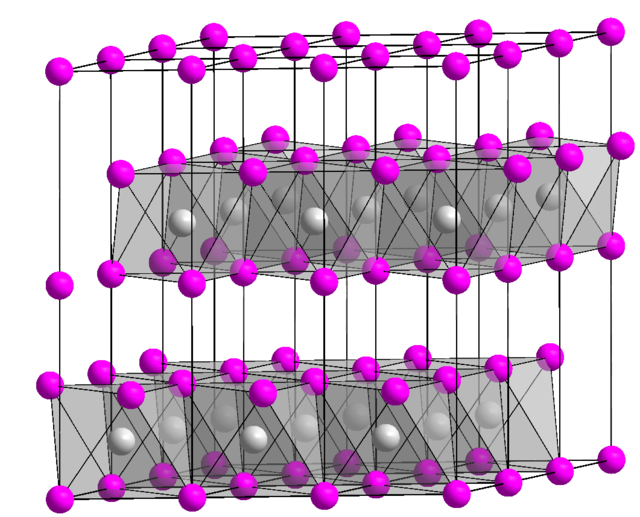
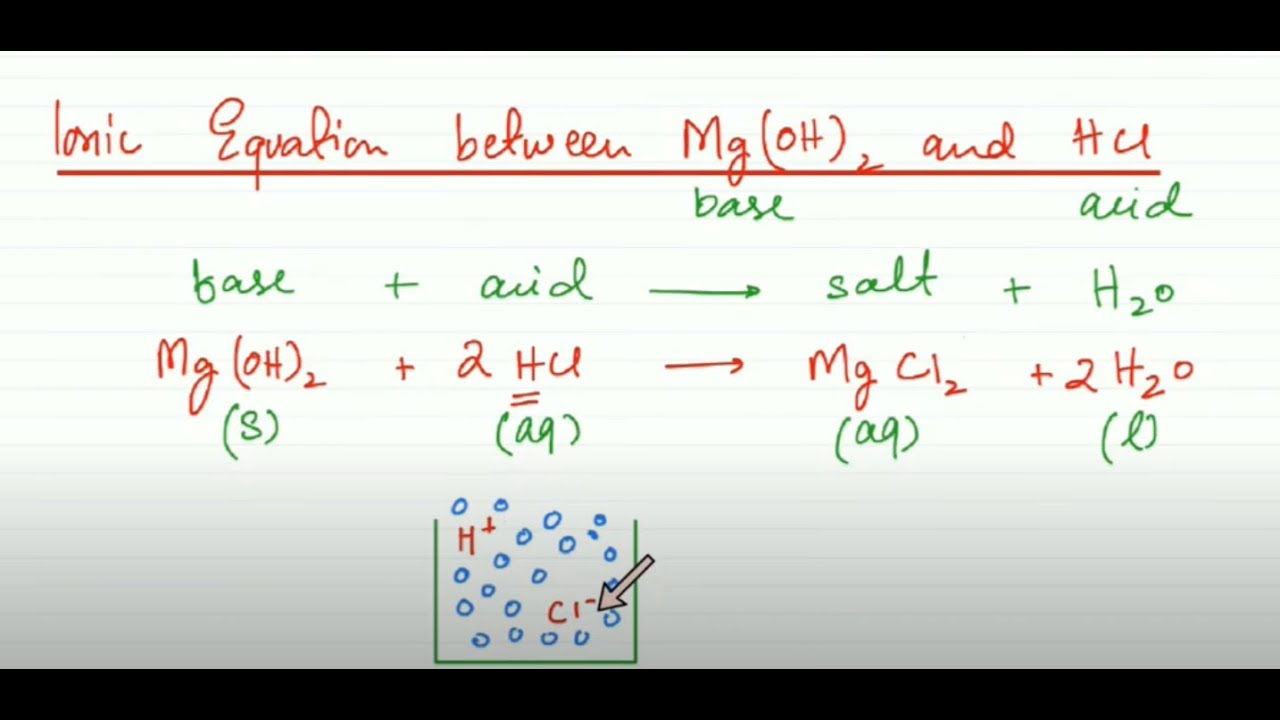
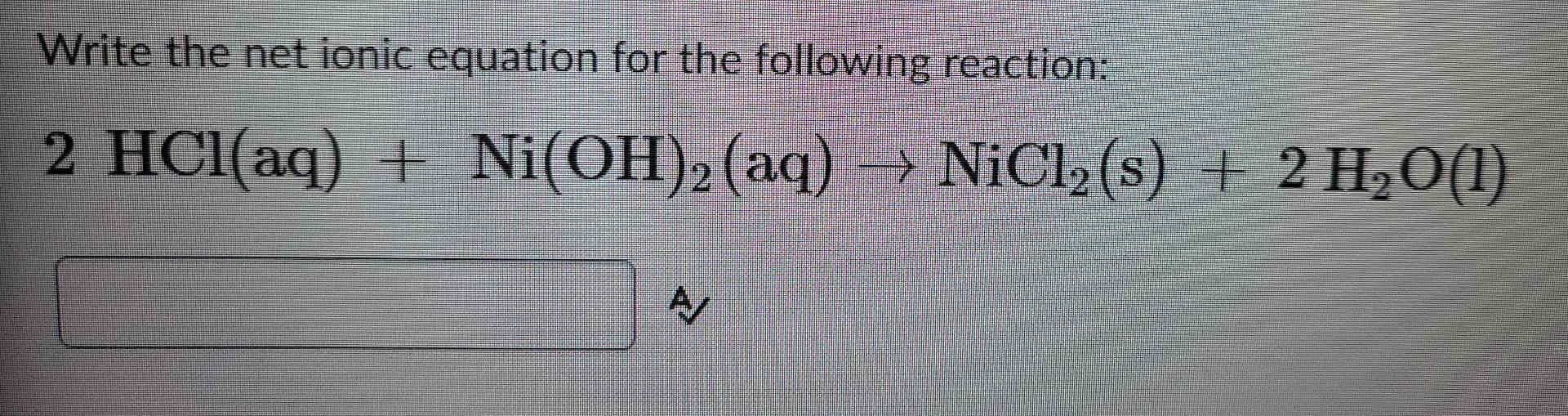Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports
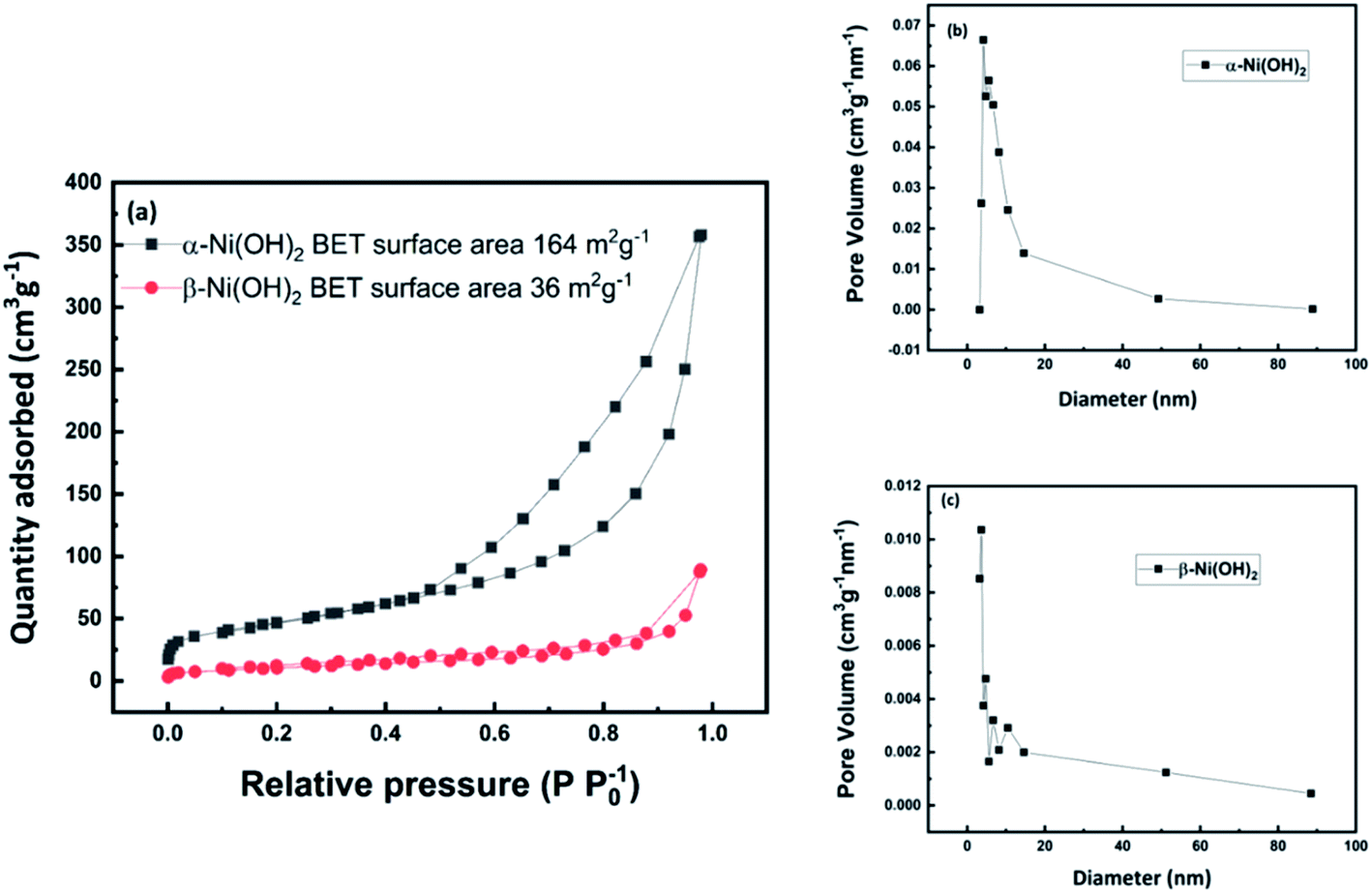
Nanoflower Ni(OH) 2 grown in situ on Ni foam for high-performance supercapacitor electrode materials - Sustainable Energy & Fuels (RSC Publishing) DOI:10.1039/D1SE01036K
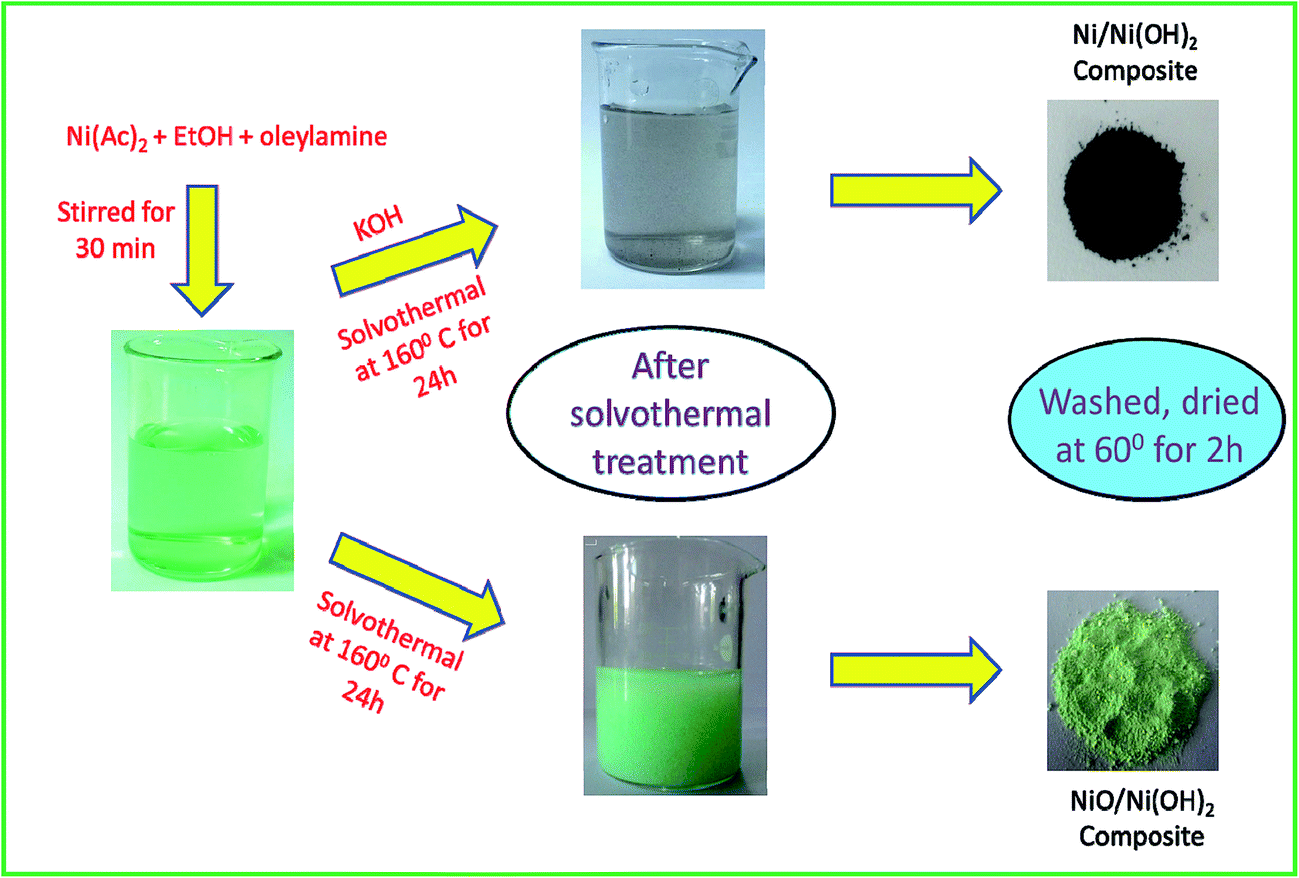
One step synthesis of Ni/Ni(OH) 2 nano sheets (NSs) and their application in asymmetric supercapacitors - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26584G
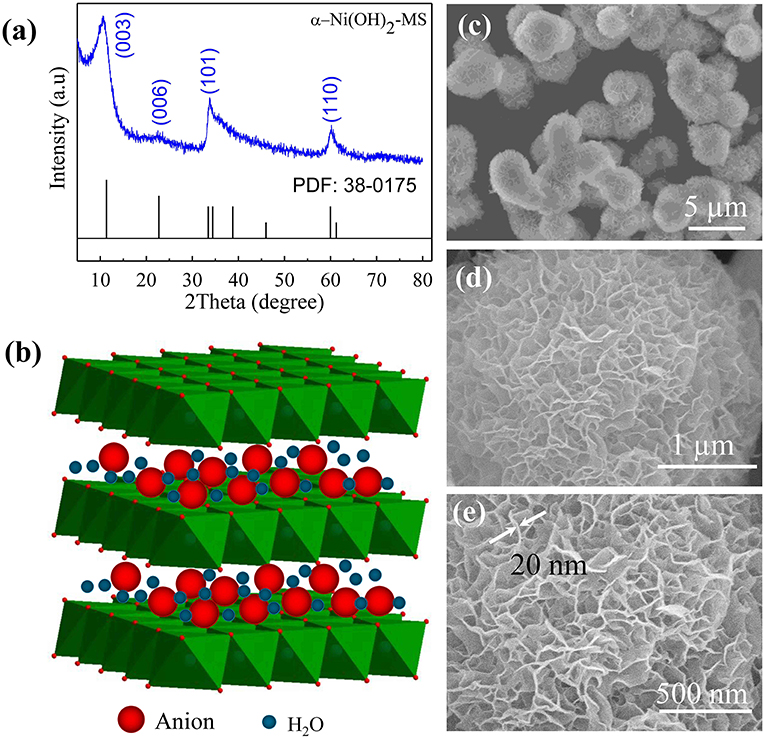
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

One material, multiple functions: graphene/Ni(OH)2 thin films applied in batteries, electrochromism and sensors | Scientific Reports

Triple Functions of Ni(OH)2 on the Surface of WN Nanowires Remarkably Promoting Electrocatalytic Activity in Full Water Splitting | ACS Catalysis