Reactions of Schiff Base‐Substituted Diselenides and ‐tellurides with Ni(II), Pd(II) and Pt(II) Phosphine Complexes - Roca Jungfer - 2020 - European Journal of Inorganic Chemistry - Wiley Online Library

Ni(II)-Catalyzed Intramolecular C–H/C–H Oxidative Coupling: An Efficient Route to Functionalized Cycloindolones and Indenoindolones | ACS Catalysis

Stereospecific/stereoselective nickel catalyzed reductive cross-coupling: An efficient tool for the synthesis of biological active targeted molecules - ScienceDirect

Synthesis, Crystal Structure, Fluorescence Property, and Theoretical Investigation of Counteranion-Introduced Ni(II) Complex with Pyridine-Appended Half-Salamo-Like Ligand | SpringerLink

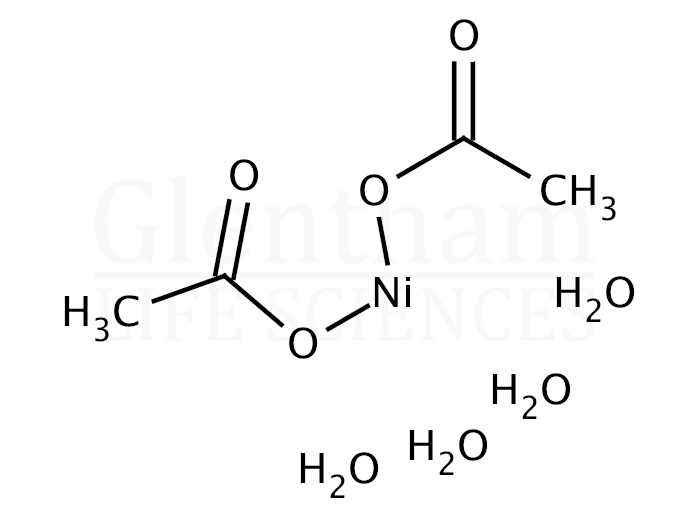
Ni(OAc)2: a highly efficient catalyst for the synthesis of enaminone and enamino ester derivatives under solvent‐free conditions - Liu - 2010 - Applied Organometallic Chemistry - Wiley Online Library
![Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fs41467-020-15837-1/MediaObjects/41467_2020_15837_Fig7_HTML.png)
Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones | Nature Communications

Carboxylation of the Ni–Me Bond in an Electron-Rich Unsymmetrical PCN Pincer Nickel Complex | Organometallics

One to Find Them All: A General Route to Ni(I)–Phenolate Species | Journal of the American Chemical Society
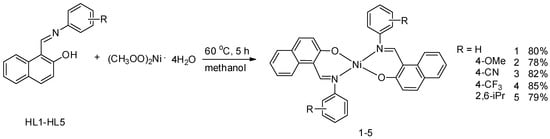
Polymers | Free Full-Text | Mononuclear Nickel(II) Complexes with Schiff Base Ligands: Synthesis, Characterization, and Catalytic Activity in Norbornene Polymerization
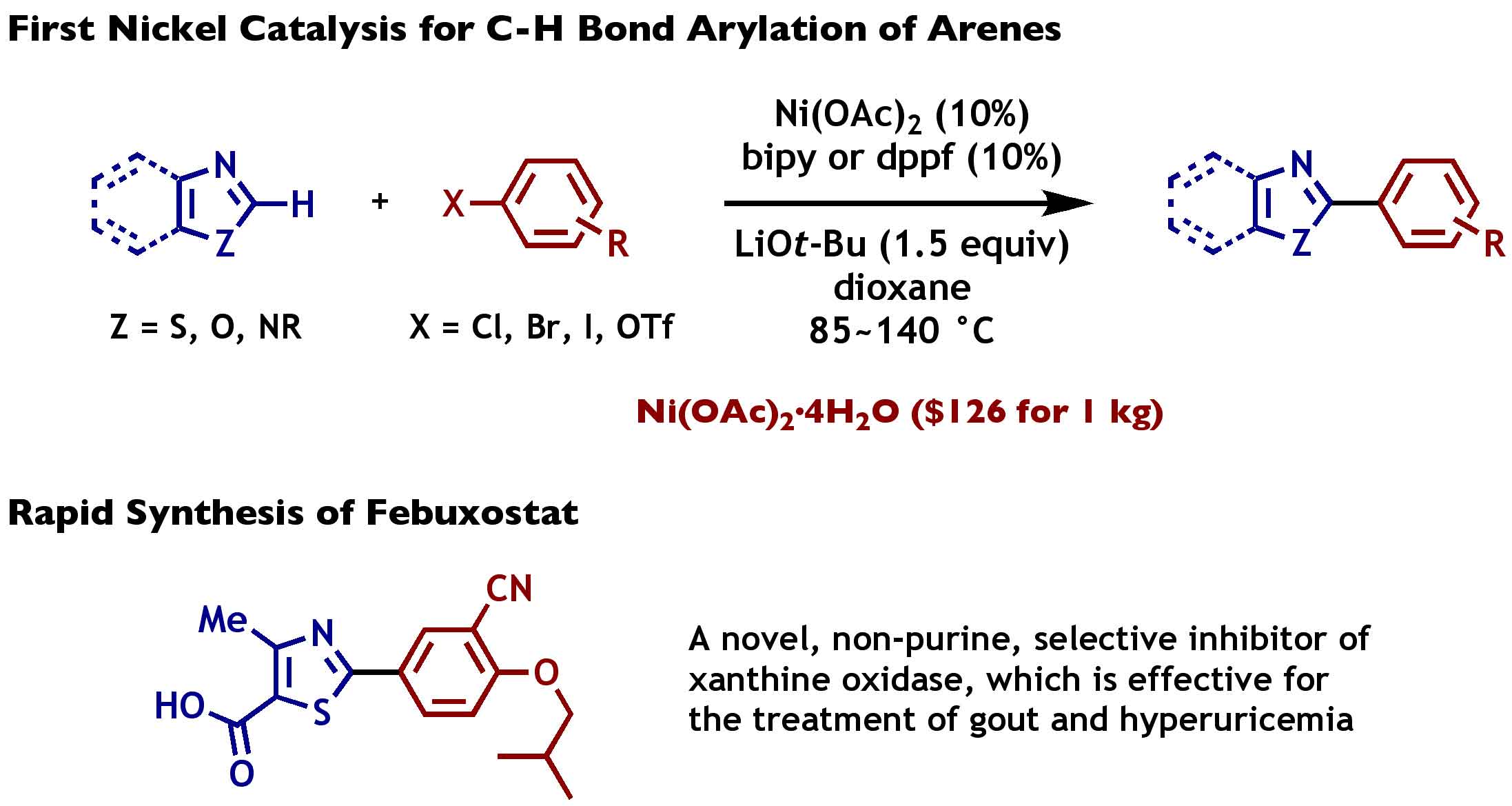
Nickel-Catalyzed Biaryl Coupling of Heteroarenes and Aryl Halides/Triflates | Itami Organic Chemistry Laboratory, Nagoya University

SYNTHESIS, SPECTROSCOPIC INVESTIGATION AND CATALYTIC STUDIES OF NICKEL(II) AROMATIC AZOMETHINE COMPLEXES | Semantic Scholar

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
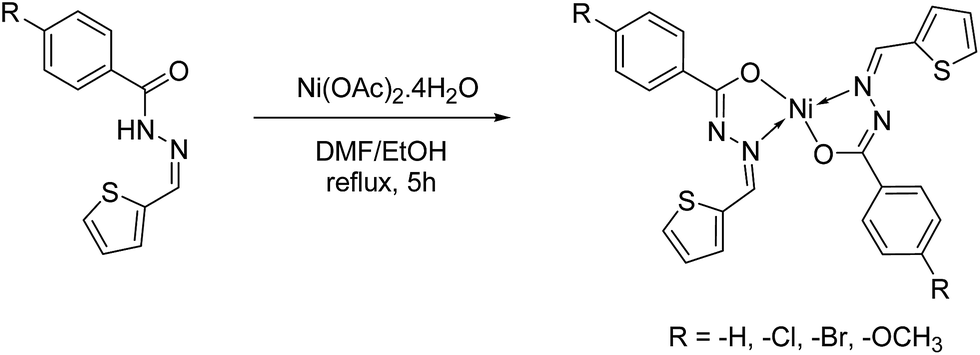
Synthesis, molecular structure and electrochemical properties of nickel( ii ) benzhydrazone complexes: influence of ligand substitution on DNA/protein ... - RSC Advances (RSC Publishing) DOI:10.1039/C5RA19530F
![Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition | Nature Communications Naked d-orbital in a centrochiral Ni(II) complex as a catalyst for asymmetric [3+2] cycloaddition | Nature Communications](https://media.springernature.com/m685/springer-static/image/art%3A10.1038%2Fncomms14875/MediaObjects/41467_2017_Article_BFncomms14875_Fig5_HTML.jpg)
![Cations of (Top) [Ni 2 L1(µ-OAc) 2 ] + (Moffat et al., 2014), and... | Download Scientific Diagram Cations of (Top) [Ni 2 L1(µ-OAc) 2 ] + (Moffat et al., 2014), and... | Download Scientific Diagram](https://www.researchgate.net/publication/327874910/figure/fig2/AS:674929695539200@1537927187016/Cations-of-Top-Ni-2-L1-OAc-2-Moffat-et-al-2014-and-Bottom-Ni-2-Me-4.png)